
TechBio platform companies are uniquely positioned to pursue many types of deals, and building your deal-making strategy has to happen earlier than you think.
I want to go over two of the most common deal types TechBio companies encounter: discovery deals (or platform deals) and asset deals. Here’s how to think through each deal, why the process is so different, and what you should expect over the course of the deal-making process for each one.
The Difference Between a Discovery Deal and an Asset Deal
An asset deal is about a partner licensing a specific product (molecule, antibody, gene delivery vehicle etc).
A discovery deal is about creating a partnership to pursue a new avenue of inquiry using your platform or technology (that may eventually lead to an asset).
Early stage platform companies are far more likely to see discovery deals than asset deals. But you should know the architecture of both. Think of an asset deal as negotiating around something of known value. The discovery deal is about forming a long-term collaborative relationship en-route to something uniquely valuable.
The Asset Deal
Pharmaceutical companies make a large amount of revenue from a small amount of drugs, so each drug is part of a greater strategy. They need to fill up their pipeline, which they can do internally through R&D or, if they need to move faster or want to mitigate risk, through the in-licensing of a later stage asset.
In September 2022 the FDA approved Bristol Myers Squibb’s Sotyktu without any additional safety warnings (a fairly off-putting feature that is common to Sotyktu’s predecessors). This sent a signal to the market that TYK2 inhibitors have significant potential for autoimmune conditions. As a result, we saw an explosion of research activity (at startups and inside pharma) as companies looked to identify their own TYK2 inhibitor.
It wasn’t long until we saw huge deals going through around these assets. In December 2022, Takeda purchased Nimbus’ late-stage TYK2 inhibitor for $4 billion in cash up front. (The biggest single asset deal in Takeda’s history).
Takeda wanted a TYK2 inhibitor. Nimbus had a late stage candidate. Deal done. We don’t know the intimate details of this deal, but the concept of a TYK2 inhibitor drug had already been vetted. Nimbus just had to prove that their drug was the right fit for Takeda.
The key here is that pharmaceutical companies often know exactly what they’re looking for when it comes to an asset deal. You don’t have to sell the concept, you have to sell your asset compared to a competitor.
The Discovery Deal
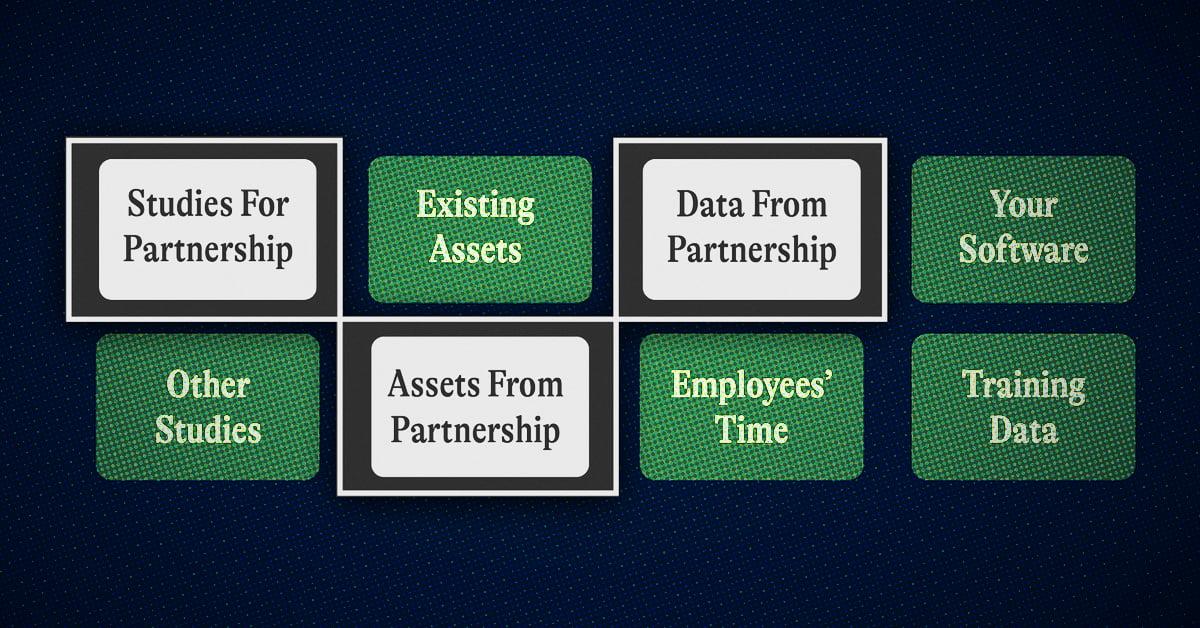
Discovery deals are all about co-development. Each party has something that the other needs. Maybe that’s a new molecule + a delivery mechanism. Maybe you can help a pharmaceutical company generate, sort, and understand their data en-route to the development of a new asset.
You’re signing up to go on a journey together, with the understanding that you’ll probably unlock unique value along the way – even if you can’t perfectly anticipate where that value will come from.
It’s up to you as the founder, and the one who knows their technology inside and out, to paint a potential picture of how this journey might unfold. Just ideating on this potential work plan can take 3 to 6 months. And at the end of the process, these deals become the length of short novels
That’s what makes the platform deal so difficult: the logic tree for making these deals grows 10x more branches. You have to agree, in advance, about what you want for what, as well as who is responsible for, and the subsequent owner of, which parts of this research and discovery process.
I was recently at an event with major stakeholders in Techbio and pharma in Boston. One of the top pieces of advice for deal-making was: find some passion for legal documents. They’ll make or break your business. That’s especially true when it comes to structuring discovery deals.
If you structure these deals well, you can gain an edge on competitors, and gain valuable validation data. But be very careful with these deal structures, because you can end up losing a lot of your future value if you don’t comprehensively carve out what you’re willing to partner on (or even give up entirely), and clearly define what you’re getting in return.
Some of the things you should think through:
- Targets to pursue
- Indications of interest
- Model organisms you’ll be using, if any
- Timeline
- Protocols to be used
- What you get from the partner:
- At minimum, you shouldn’t be paying out of pocket for these experiments, your larger biotech or pharma partner should be sure that you aren’t digging into your runway for this deal
- Ideally you can leverage more than just upfront cash. You can also gain access to expertise and resources from your pharma and biotech partners such as FTE’s working on this and ideating on how to set up experiments
- If there is equity involved at this early stage, agree to how this is handled since adding an external company to your cap table is a sensitive move (terms/timelines for selling shares etc)
- Data as an asset (relevant for AI drug discovery deals)
- IP protection and trade secrets around the platform (an AI model, for example)
- Is there a clear link from your platform to the proposed end asset?
- Who is your internal champion and partner at the beginning, middle, and end of this deal.
Guiding Principle: Don’t give away too much
One of the biggest mistakes you can make during this process is giving away too much.
Head of NFXBio Omri Amirav Drory has emphasized that platforms don’t have to sell their future for the present. To avoid doing that, make sure that what you are selling (or offering to a partner) is extremely well defined.
As you draft a discovery deal document, think of your goal being Litchom לתחום – or, in English, setting a border or boundary. Inside that border are the parameters of your collaboration: which aspects of the platform are being used, what studies are being done, who gets any potential asset rights later. Anything outside of that border belongs to you and is not within the scope of the deal you are setting up.
Closing the Deal: The Psychology of Discovery Deals and Asset Deals
The mindsets behind closing asset and discovery deals can be radically different.
Sometimes pharmaceutical companies have completely different teams dedicated to each type of deal, so it’s up to you to find the right person for the deal you want to make. Sometimes one person handles all types of deals.
If you understand the mindset of the person you’re dealing with, you’ll be better able to gauge how long the deal should take, and whether the interest on the other side is genuine.
The Asset Mindset: Quick Strategic Wins
Asset deals are more likely to contain elements of speed and strategy. You set the terms, you negotiate, it goes through.
Speed is an indicator of interest. If a pharmaceutical company wants this deal to happen, they will make it happen.
Look for evidence of urgency. They can hire external counsel to speed things up, take calls at odd hours etc.
The Discovery Mindset: Scientific Passion
Discovery deals are fueled by curiosity, seeing the long game, and a deep love of science.
You don’t sign up for an asset deal that could unfold over months or years unless you’re genuinely excited about pursuing a new avenue of scientific discovery.
You must demonstrate why you fit with pharma’s overall strategy, know the disease landscapes, and understand their goals. But the softer side of this is gauging whether your partner feels genuinely excited about you, your platform, and what the future holds for this collaboration.
Gauging this is far trickier. Why? No one wants to admit this, but emotions play a far larger role in discovery deals than you might think.
You can’t manufacture this type of relationship overnight. That’s one of many reasons laying the right groundwork for your deal months, even years, ahead of time is so critical. (Find our guide to building your dealmaking network here).
You have to learn the culture inside each company to really understand whether you’re moving in the right direction. For example, there are companies where you really need a BD person to push upper management to consider your deal, in others, you need buy-in from a head of a certain therapeutic area.
That said, you can look for certain rough indicators that might indicate genuine interest. Here, speed is less of a factor (as we mentioned earlier these deals take time). Instead, your partner should be responsive to your inquiries. You should notice that you’re being introduced to more people at your target company. We call this ‘moving up the chain of intros.’
Don’t Fear the Feasibility Study
One of the key parts of any discovery deal is a feasibility study.
This process causes so much anxiety for many founders. It’s not because they don’t believe in the reproducibility of their science, but often they fear a loss of control. They fear a loss of control over their IP, over the protocol of their study and that, should anything random go wrong, it will reflect badly on them and make future deals more difficult.
First: don’t worry about handing over IP. Pharma companies aren’t going to steal your IP during a feasibility study. Just get over this and realize it isn’t how things work.
Second: You should be able to see the protocol of your feasibility study in advance in detail, and then work together on how the protocol should look, and why. This is actually an opportunity to learn and improve from teams with deep experience in designing these studies.
Co-designing protocols should be in your agreement. Some companies may even agree to come to your lab and do feasibility studies there.
Third: These studies should not hurt you financially in any way. Pharma should be paying for them. Rule #1 of dealmaking: a bad deal is one you pay for.
If you have a solid agreement in place, you can flip your perspective on feasibility studies: these are incredible opportunities.
So what’s the worst case scenario? The worst case scenario is that, even with all protocols checked, and no money spent, your approach isn’t validated. In that case, you are saving yourself a world of hurt.
If you have a problem, it’s better to know early on, before there are more stakeholders involved. It gives you time to adjust course.
What’s the best case? You get top quality external validation. And it leads to a future partnership.
Stay in the Driver’s Seat
So many scientist-Founders fail to start great companies because they’re afraid of the business side of things. If you can complete your PhD, or discover defensible magic, you can learn to read legal documents, understand biz dev, and run a business.
You can, and should, hire or partner with people who are great business operators. But to unlock all of your potential as a scientist-founder you have to see the whole picture – both business and science.
Understand the broad strokes of the deals you’re making, the psychology of business development, and where your technology fits in.
Especially when it comes to discovery deals, no one understands the potential of your technology better than you do.
With just a bit of preparation, you’ll stay in the driver’s seat – where you belong.
As Founders ourselves, we respect your time. That’s why we built BriefLink, a new software tool that minimizes the upfront time of getting the VC meeting. Simply tell us about your company in 9 easy questions, and you’ll hear from us if it’s a fit.